The bioresorbable Absorb scaffold considered the holy grail of coronary intervention is now being pulled out from the commercial market in Europe. The stunning news is the culmination of sticky data emerging from recent randomized trials assessing the bioresorbable vascular scaffold (BRS), that have revealed that with this generation of BRS both efficacy and safety are questionable.
The ABSORB II trial reported a significantly higher rate of target vessel myocardial infarction last year. The ABSORB III trial further dampened enthusiasm by showing significant increase in target lesion failure. The last randomized trial to be published was actually terminated early because of increased stent thrombosis including late stent (BRS) thrombosis.
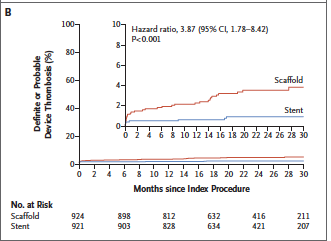
The AIDA (Amsterdam Investigator Initiated Absorb Strategy All Comers Trial) investigators randomly assigned 1845 patients to either receive a BRS (924 patients) or a metallic stent (921). Median follow up was 707 days. Target vessel failure (a composite of cardiac death, target vessel MI, or target vessel revascularization) occurred in 105 patients with BRS and in 94 patients in the stent group. Target vessel MI occurred in 48 patients in the BRS group but in only 30 provided metallic stents. Definite or probable stent thrombosis occurred in 31 patients in the scaffold group as compared with 8 patients in the stent group (3.5% vs. 0.9%; p<0.001). This was a single blind, multicenter, investigator initiated non-inferiority trial.