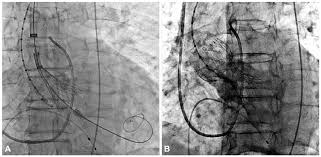
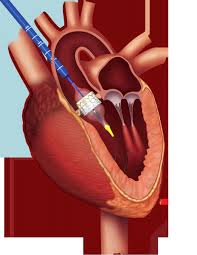
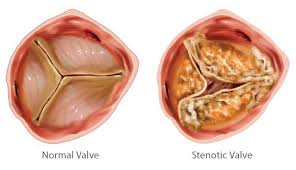
Transcatheter aortic valve replacement (TAVR), performed for the first time in 2002, is now considered a suitable alternative to surgical valve replacement (SAVR) in high-risk patients with severe symptomatic aortic stenosis. The PARTNER trial was the first to directly compare TAVR to SAVR head to head in high surgical risk patients; at 1 year TAVR performed with balloon expandable Edwards-Sapien valve was non- inferior to SAVR (24% vs. 27%). This was maintained at 5 years follow up (68% vs. 62%).
Recently the CoreValve US Pivotal trial comparing TAVR with the self expanding CoreValve with SAVR has reported lower all cause mortality in the TAVR arm at 1 year (14% vs. 19%). The difference was no, longer significant at 3 years (33% vs. 39%). It is reassuring that TAVR with the CoreValve has similar rate of mortality as with SAVR. The patients chosen for the study had to have an estimated 30-day mortality of 15% and combined mortality and morbidity < 50%. The Pivotal trial randomised 797 patients with severe aortic stenosis to TAVR and SAVR. The investigators concluded that patients with severe aortic stenosis at increased risk for surgery had improved 3-year clinical outcomes after TAVR compared with surgery. Aortic valve haemodynamics were better with TAVR without differences in structural deterioration.
At the end of 1-year stroke rates were less with TAVR versus SAVR and continued to be lower with TAVR at 3 years (13% vs. 19%). In the PARTNER trial at 3 years, stroke rates of TAVR and SAVR were 8% versus 9%.
The rates of permanent pacemaker implantation were highest in the first month for TAVR versus SAVR (20% vs. 7%), and persisted at 28% versus 15% at 3 years. Almost 1 in 2 patients with a CoreValve will need a permanent pacemaker by 3 years because 22% of patients already had had a permanent pacemaker at the start of the trial. This will add to the cost of treatment with TAVR.
Most importantly valve performance and hemodynamics were maintained over 3 years of follow-up. Mean gradient and effective orifice area were better with TAVR than SAVR. There has been no report of valve destruction/degeneration or valve thrombosis with either the CoreValve or the Edwards-Sapien valve. Longer follow-up is needed to assess durability of TAVR bearing in mind that surgical bioprosthetic valves have a median lifespan of 10-15 years.
Moderate to severe aortic regurgitation was observed in 5-8% patients in the TAVR arm. This was mostly paravalvular in nature.
It must be also kept in mind that the CoreValve as also the Edwards-Sapien valves have improved in structure and usability. The current commercially CoreValve has a smaller sheath size, extended sealing skirt and can be recaptured. Both TAVR and SAVR are good options for treatment of complex aortic stenosis patients.
The PARTNER 2 trial has also been reported that TAVR is the treatment of choice in most patients with aortic stenosis who are at high risk for early death and complications with SAVR, particularly if the patient permits the femoral approach. The PARTNER 2 trial compared the second generation SAPIEN XT TAVR system with SAVR in intermediate risk patients. Patients had to have aortic valve area of <0.8 cm2 and mean aortic valve gradient >40 mm Hg or peak jet velocity >4 m/s.
Patients with bicuspid valve, aortic annulus diameter <18 mm or > 27 mm (echo or CT), LVEF <20% were excluded. More than 2000 patients were randomized to TAVR or SAVR.
At 2 years, TAVR using SAPIEN XT and SAVR were similar for the primary end point of all cause mortality or disabling stroke (17% vs. 18%). But in the transfemoral subgroup (76% of patients), TAVR using SAPIEN XT significantly reduced all cause mortality or disabling stroke versus surgery (17% vs. 20%). TAVR resulted in larger aortic valve area than surgery but with lesser acute kidney injury, new onset atrial fibrillation, and severe bleeding. The investigators of PARTNER 2 concluded that TAVR and SAVR had similar clinical outcomes regarding death or stroke up to 2 years and equal lessening of cardiac symptoms.
The cost of TAVR in India however remains exorbitant and the procedure therefore is currently out of the reach of most patients.