Heart failure is a debilitating and life threatening disease in which the heart is unable to adequately pump blood around the body leading to symptoms of breathlessness, fatigue and fluid retention. About half of heart failure patients have reduced ejection fraction when assessed by 2D echocardiography.
Systolic heart Failure continues to torment millions of patients across the world despite enormous progress in drug treatment over the last 30 years. There was no drug capable of cutting down death rate available in the 1980’s when my Mum was suffering from breathlessness, swollen feet and tiredness due to ischemic cardiomyopathy. The CONSENSUS study reporting mortality benefit with enalapril got published just then and I desperately acquired the medicine for my Mum but she sadly found it too toxic. The smallest dose would nauseate her. I was crushed, for there was no alternative then. George Orwell died of TB in the 1940’s; streptomycin had been discovered but Orwell simply could not tolerate the antibiotic despite getting hold of it in the United States. It seems incredible now but there was no treatment for heart failure till the mid 1980’s.
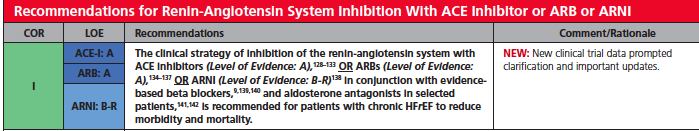
The CONSENSUS (1987) and SOLVD-Treatment (1991) trials recorded reduced overall mortality by 16 to 40% with enalapril an angiotensin converting enzyme (ACE) inhibitor. Enalapril was also shown to be better than the combination of hydralazine and isosorbide dinitrate in the V-HeFT II trial. In 1992 the SOLVD-Prevention trial showed enalapril could also reduce rate of hospitalization for heart failure in asymptomatic patients with reduced ejection fraction. ACE inhibitors would become the bedrock of heart failure treatment for the next 30 years.
The Val-HeFT trial established angiotensin receptor blocker (ARB) therapy could also be used for heart failure, but because ARB’s are not superior to ACE inhibitors, ARB’s are reserved for patients who cannot tolerate ACE inhibitors. An ARB interferes with the action of angiotensin II (that causes vasoconstriction).
Beta-blockers were next to claim their exalted status in reducing mortality in patients with heart failure. Bisoprolol, and extended release metoprolol. Carvedilol (a beta and alpha blocker) showed substantial reduction in mortality in the US Carvedilol Heart Failure Study (1996) and COPERNICUS (2001) trials.
The third group of drugs to demonstrate lowering of death in heart failure is the mineralocorticoid receptor antagonists (MRA) spironolactone and eplrenone. Spironolactone could reduce mortality by 30% in the RALES study (1999) while eplrenone also showed substantial clinical benefit in mild heart failure patients in the EMPHASIS-HF trial (2011). In the RALES trial, spironolactone reduced mortality in patients already on an ACE inhibitor and a diuretic; this was huge incremental advantage.
In 2014 a new combination drug showed further incremental lowering of a composite of cardiovascular death and hospitalization for heart failure by 20% as compared to the ACE inhibitor enalapril. The new drug named “Entresto” is a combination of valsartan and sacubitril (an inhibitor of neprilysin). Sacubitril provides additional advantage by blocking neprilysin and thereby raising levels of natriuretic peptides, which have vasodilator properties, increase sodium excretion and also aid in remodeling. There was a 5% absolute reduction in the composite clinical endpoint (down from 27% to 22%), and a 3% absolute reduction in mortality with the sacubitril/valsartan combination as compared to enalpril (17% vs. 20%). There was moreover lesser hyperkalemia, kidney impairment and cough with the combination drug. This trial was rightly called the PARADIDGM-HF trial.
The PARADIGM-HF trial included patients of heart failure in NYHA class II-IV symptoms, ejection fraction less than 40%, a BNP level >150 pg/ml or NT pro BNP > 600pg/ml. Diuretics were used by 80%, beta blockers by 93%, mineralocorticoid inhibitor by 55% in both groups of the PARADIGM HF study. Cardiac resynchronization therapy was present in about 7% of patients in both groups. The best starting dose should be 24/26 twice a day.
In the ACC 2017 meeting the investigators presented data that showed sacubitril/valsartan also lowered glycated hemoglobin (HbA1C) more than enalapril in 3778 reduced ejection fraction heart failure patients diagnosed with diabetes. The PARADIGM-HF trial studied patients with heart failure and reduced ejection in a double blind randomized manner. Approximately 40% of patients studied had diabetes. The intervention drug reduced HbA1C levels by 0.26% compared to 0.16% by enalapril. Also 29% fewer patients in the sacubitril/valsartan arm had to start insulin in order to get this level of control. The suggestion is that diabetic patients with reduced ejection fraction heart failure may need to reduce or revise their anti-diabetic treatment.
Crucially, the American College of Cardiology, the American Heart Association, and the Heart Failure Society of America have updated heart failure guidelines to recommend use of 2 new drugs in treatment of heart failure accompanied by reduced ejection fraction; the angiotensin receptor-neprilysin inhibitor (sacubitril/valsartan) and the Sino atrial node modulator ivabradine. The guidelines state these 2 drugs ‘represent a milestone in the evolution of care for patients with HF.” Sacubitril/valsartan is now recommended for use in place of an ACE inhibitor or an ARB in heart failure with reduced ejection fraction. The combination may be used as part of a regimen that includes other heart failure medications such as diuretics, mineralocorticoid antagonist, beta-blockers and isosorbide/hydralazine. For patients with symptomatic class II or III heart failure with reduced ejection fraction who tolerate an ACE inhibitor or ARB, the guidelines recommend replacing the existing ACE inhibitor or ARB with a sacubitril/valsartan to reduce morbidity and mortality. However the American Academy of Family Physicians is not ready to endorse the guidelines set by the 3 specialty heart societies. The Academy of Family Physicians insists on more randomized trials with sacubitril/valsartan to confirm safety and efficacy before endorsing the new drug