There are currently millions of patients with diabetes in India, which is rapidly becoming the world’s capital for diabetes largely due to the horrendous life styles adopted by the general population. India is home to more than 60 million diabetics (95% are people with Type 2 diabetes).There will be more than a 100 million by the year 2030. Almost 2.1% of the Indian GDP is spent in treating diabetes and its complications, including myocardial infarction, heart failure, stroke, blindness, kidney failure and amputation. The average Indian loves his or her food and the only exercise done is sitting up watching a game of cricket on TV.
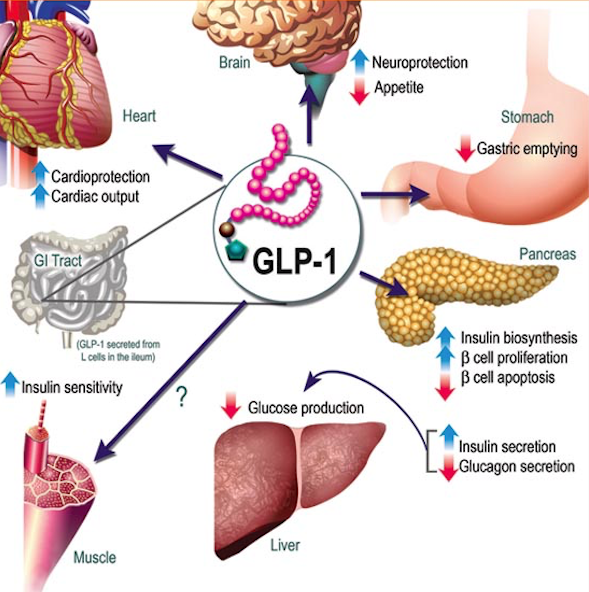
Mercifully almost all drugs required to treat diabetes are available at reasonable rates in India, including basal insulin, metformin, sulfonylureas, and even the new sodium glucose transport inhibitors. Novo Nordisk, a leading global healthcare company with special interest in diabetes is set to launch its latest product, a cretin mimetic, called liraglutide. Liraglutide named Victoza by the company is a synthetic injection that mimics a glucagon like peptide analogue (GLP-1). Victoza or liraglutide is a once a day injection that aims to reduce raised blood sugar in blood by stimulating natural production in the pancreas. It must be of course used along with a healthy diet and exercise program. Liraglutide is not an insulin product but 97% similar to GLP-1. Liriglutide also prevents absorption of sugar, delays gastric emptying and reduces appetite, and counters the glucose releasing effect of glucagon on the liver. It is well known to reduce weight, and has the advantage of not triggering hypoglycemia.
Currently injection liraglutide is the favorite property of Novo Nordisk, which fetched them 1.7 billion US dollars global sales in 2012. There have been however serious concerns of associated pancreatitis and pancreatic cancer with all incretin mimetics including liraglutide. The makers of Byetta (exanatide; a GLP-1 analogue) and Januvia (sitagliptin; a DPP-4 inhibitor) are facing numerous lawsuits that claim these drugs cause pancreatic cancer. The FDA had been petitioned by a health NGO to remove liraglutide from the market (Dr. Sidney Wolfe; director of ‘Public Citizen’). The FDA decided that withdrawal of liraglutide was not warranted but that safety monitoring of the drug would continue.
In the meantime the New England Journal of Medicine has published the LEADER Trial, which has concluded that injection liraglutide given once daily at a dose of 1.8 mg over a period of (median) 3.5 years provided considerable cardiovascular benefits to patients with diabetes. The LEADER Trial in a double randomized manner assigned more than 9000 patients with Type 2 diabetes to standard diabetes treatment plus liraglutide or standard diabetes treatment plus placebo injection. The median daily dose of liraglutide was 1.78 mg; the demographic and clinical characteristics of the patients were alike in the two groups. Of the 9340 patients the majority (81%) had established cardiovascular disease while 72% had chronic kidney disease. Duration of diabetes at baseline was almost 13 years and mean glycated hemoglobin was 8.7%.
The composite primary outcome (consisting of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was lower in the liraglutide group by an absolute 1.9% (from 14.9% to 13%; p =0.01) by liraglutide. Death due to cardiovascular cause was lowered an absolute 1.3% (from 6% to 4.7%; p=0.007). There was no significant difference in nonfatal myocardial infarction or nonfatal stroke with luraglutide. There was a difference of only 0.1% in non-cardiovascular deaths (from 3.6% to 3.5%; p=0.66).
We must now focus on the much-touted ‘significant reduction’ in cardiovascular mortality by 1.3%. Another way of putting this down would be that on standard diabetic treatment, 94% of patients were alive after a median of 3.5 years while liraglutide enabled 95.3% to be alive by 3.5 years. This also means that 77 diabetic patients would have to be treated with liraglutide above standard diabetic medication for 3.5 years to save one life. In the US, the cost of a month’s treatment with liraglutide is approximately $540. A single patient with diabetes would have thus to spent more than $22,000. The cost of treating 77 patients to save one life would create a bill of $1.694 million!
The lay press has been conned into reporting that there was risk of all cause mortality and cardiovascular mortality by 15% and 22% respectively. Reductions in events in relative terms always amplify the efficacy of a drug.
Now lets check the adverse effects reported in the LEADERS Trial. Any adverse event was increased by an absolute 1.5% (from 60.8% to 62 % in the group treated with liraglutide. There was significant increase in acute gall stone disease with liraglutide (1.9% to 3.1%; p < 0.001). Any adverse event leading to permanent stoppage of trial regimen occurred significantly more times with liraglutide (from 7.3% to 9.5%; p<0.001). There was significant increase in nausea, vomiting, diarrhea, abdominal pain, and abdominal discomfort.
There were moreover more benign and malignant neoplasms with liraglutide as compared to placebo. Pancreatic cancer was seen in 13 patients in the liraglutide group versus 5 in the placebo group. Retinopathy was also more often seen in the liraglutide group.
All of the above was achieved by lowering glycated hemoglobin by a mere 0.4% in the group treated with liraglutide. The authors of the paper are unable to explain the causes for the reduction in cardiovascular mortality in the trial. There is absolutely no explanation provided in the discussion section. Systolic blood pressure was lessened by 1 mm Hg in the liraglutide while heart rate was increased by 3 beats per minute on an average.
I am not convinced by the paltry lowering of deaths with liraglutide in patients with Type 2 diabetes at the cost of millions of dollars. It is more than probable that equivalent lowering of death can be achieved by ensuring that a diabetic patient eats 100-150 calories less in a day or walks briskly for another 8-10 minutes a day. Which begs the question; what makes the New England Journal of Medicine (NEJM) publish industry sponsored papers that manifestly tend to exaggerate clinical efficacy, while downplaying serious adverse effects of the drug being researched. The paper clearly states in the results and table provided that there was insignificant reduction in nonfatal myocardial infarction (by only 0.8%) and stroke (by a mere 0,4%) and yet the conclusions in the abstract brazenly informs the reader that, “nonfatal myocardial infarction, or nonfatal stroke among patients with type 2 diabetes mellitus was lower with liraglutide than with placebo.” There are,among the authors , 4 employees of the company making Liraglutide and 11 academic researchers who have received some or the other form of funding from the company.
The average Indian would probably suffer a heart attack if told that he would have to spend more than $7,000 a year on a drug, which has marginal clinical cardiovascular benefit. He would be petrified if told that the same drug carries the potential for increasing the risk of cancer. We therefore need a realistic reduction in the pricing of liraglutide ,and crucially, wait for more long term randomized trials confirming beneficial cardiovascular effects of liraglutide